Many students often ask a simple but confusing question: “Is H2O polar or nonpolar?” Because water is something we use every day, it may feel strange that its molecule has a special behavior. People mix up the terms because polar and nonpolar sound scientific and difficult. But don’t worry — in this guide, everything is explained in easy, child-friendly language.
By the end of this article, you will understand the meaning of polar, the meaning of nonpolar, and the difference between polar and nonpolar in a simple way. You’ll also learn when to use each term, how to identify polarity quickly, and how to avoid common mistakes. This explanation is designed so even a 4th-grade student can understand it perfectly.
1. What Does “Polar” Mean?
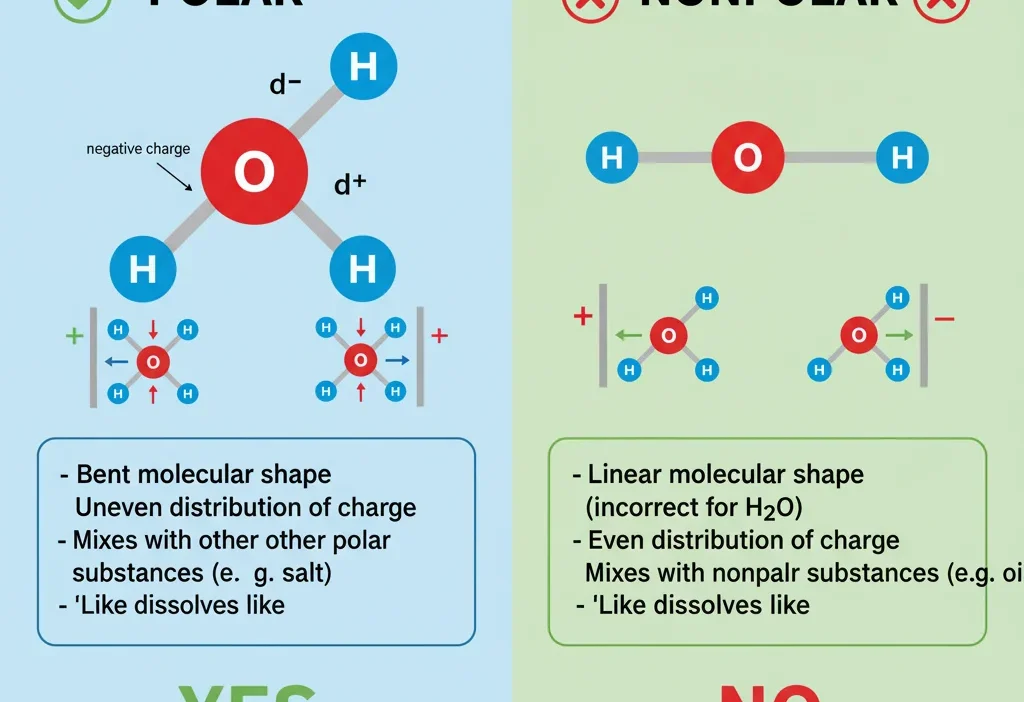
A polar molecule is like a magnet.
It has two sides that behave differently:
- One side is a little positive.
- One side is a little negative.
This happens because the atoms inside the molecule do not share electrons equally.
Simple Examples of Polarity
- Water (H2O)
- Ammonia (NH3)
- Hydrogen fluoride (HF)
Easy Story Example
Imagine two friends sharing a chocolate bar. One friend takes more chocolate than the other. The sharing becomes unequal.
That is how electrons behave in a polar molecule — one atom “pulls” harder than the other.
2. What Does “Nonpolar” Mean?
A nonpolar molecule shares electrons equally.
This means:
- No positive side
- No negative side
- Same behavior on every side
Simple Examples of Nonpolarity
- Oxygen gas (O₂)
- Methane (CH₄)
- Nitrogen gas (N₂)
Easy Story Example
Think of two friends each sharing the chocolate bar perfectly. No one takes more.
That is a nonpolar molecule — everything is fair and equal.
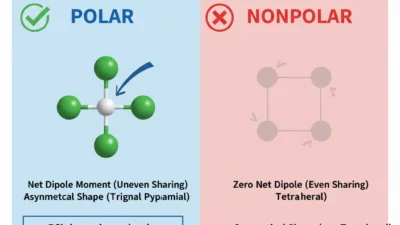
The Key Difference Between Polar and Nonpolar
Now let’s look at the main difference so you can clearly answer: Is H2O polar or nonpolar?
Comparison Table: Polar vs Nonpolar
| Feature | Polar Molecule | Nonpolar Molecule |
|---|---|---|
| Sharing of Electrons | Unequal | Equal |
| Charge Distribution | Has positive & negative ends | No charged ends |
| Shape Effect | Bent or uneven shapes often cause polarity | Symmetrical shapes stay nonpolar |
| Example | H2O (water) | CO₂, O₂ |
| Attraction | Attracts other polar molecules | Attracts other nonpolar molecules |
Quick Tip to Remember
- Polar = “Pulling apart”
- Nonpolar = “No pulling”
Is H2O Polar or Nonpolar? The Correct Answer
H2O is polar.
Here’s why:
- Oxygen pulls electrons more strongly than hydrogen.
- The shape of water is bent, not straight.
- This creates a positive side (hydrogen side) and a negative side (oxygen side).
Because the sharing is unequal, water becomes polar.
Common Mistakes and How to Avoid Them
Many learners make simple mistakes while studying polarity. Here are the most common ones.
Mistake 1: Thinking shape doesn’t matter
Wrong:
“H2O should be nonpolar because it has two hydrogens.”
Correct:
Shape does matter. Water is bent, so its sides are not equal.
Mistake 2: Believing equal atoms always mean nonpolar
Wrong:
“CO₂ must be polar because oxygen pulls harder.”
Correct:
CO₂ is linear (straight), so the pulls cancel out and make it nonpolar.
Mistake 3: Assuming polarity is too “scientific” to understand
Wrong:
“I can’t learn polarity; it’s too hard.”
Correct:
Polarity just means fair vs unfair sharing of electrons — that’s it!
When to Use the Word “Polar”
Use polar when:
- The molecule has a positive and negative end
- Electrons are shared unequally
- The shape is bent or uneven
- One atom pulls harder than the other
Example Sentences
- Water is a polar molecule.
- Salt dissolves well in polar liquids.
- Vinegar mixes with water because both are polar.
- A polar molecule acts like a tiny magnet.
- Sugar also dissolves in polar liquids like water.
When to Use the Word “Nonpolar”
Use nonpolar when:
- Electrons are shared equally
- The molecule has no positive or negative side
- The shape is equal or balanced
- No atom pulls harder
Example Sentences
- Oil is nonpolar, so it doesn’t mix with water.
- Oxygen gas (O₂) is a nonpolar molecule.
- Candle wax is nonpolar, so it stays solid.
- Nonpolar molecules prefer other nonpolar molecules.
- Carbon dioxide is nonpolar because its shape is straight.
Memory Hack
Think of nonpolar as “NO poles.”
If a molecule has no separate sides, it’s nonpolar.
Quick Recap: Polar vs Nonpolar
- Polar = unequal sharing of electrons
- Nonpolar = equal sharing
- H2O is polar because oxygen pulls harder and the shape is bent
- Nonpolar molecules look balanced and even
- Shape + pulling strength decide polarity
Advanced Tips (Optional)
1. History of the Terms
The words polar and nonpolar come from the idea of “poles,” just like the North Pole and South Pole of Earth. Polarity means having two different sides.
2. Formal Writing Tip
In essays or exams, always explain polarity with two points:
- Electronegativity difference
- Molecular shape
This shows clear scientific understanding.
3. Polarity in Everyday Life
- Soap works because one end is polar (loves water) and the other is nonpolar (loves oil).
- This helps scrub away dirt from skin and clothes.
Mini Quiz: Test Your Understanding
Fill in the blanks:
- H2O is a ______ molecule.
- ______ molecules share electrons equally.
- A polar molecule has two different ______.
- CO₂ is ______ because its shape is straight.
- Shape is important when deciding ______.
5 FAQs (For Featured Snippets)
1. Is H2O polar or nonpolar?
H2O is polar because electrons are shared unequally and the shape is bent.
2. Why is water polar?
Water is polar because oxygen pulls electrons more strongly than hydrogen.
3. What is the difference between polar and nonpolar?
Polar molecules have unequal sharing; nonpolar molecules share equally.
4. Is CO₂ polar or nonpolar?
CO₂ is nonpolar because its straight shape cancels out charges.
5. Does shape decide polarity?
Yes. Shape plays a major role in making a molecule polar or nonpolar.
Conclusion
Understanding is H2O polar or nonpolar becomes simple when you know the meaning of the words polar and nonpolar. Water is polar because its electrons are shared unequally, and its shape is bent. With the easy tips, examples, and explanations in this guide, anyone — even a beginner or child — can identify polarity correctly.
Keep practicing with everyday examples, and soon you’ll feel confident in explaining polarity to others too.

Marianne Solace is a lifestyle and personal-growth writer for WordContrast.com. Her work blends inspiration with practicality, offering thoughtful insights on wellness, creativity, and mindful living. When she’s not writing, Marianne enjoys journaling with a cup of coffee, exploring art museums, and helping others find balance through the written word.